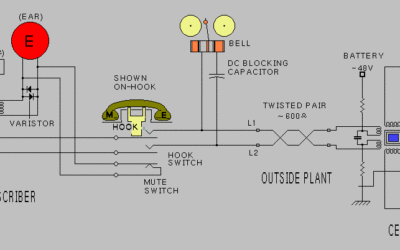
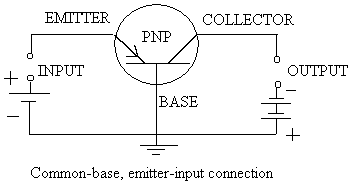
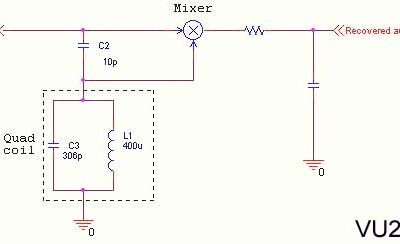
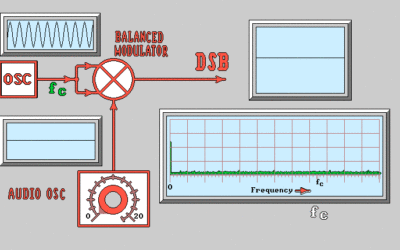
Primary Cell:
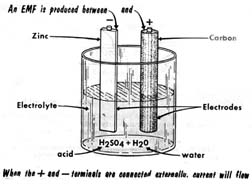 When dissimilar materials like zinc and carbon(or copper used in place of carbon)were immersed in a bath of acid solution(suphuric acid diluted in water), chamical action produced an electromotive force between the zinc and the carbon. The materials immersed in the acid were called the electrodes, with the zinc being negative electrod and other(carbon/copper)being the positive electrode.
When dissimilar materials like zinc and carbon(or copper used in place of carbon)were immersed in a bath of acid solution(suphuric acid diluted in water), chamical action produced an electromotive force between the zinc and the carbon. The materials immersed in the acid were called the electrodes, with the zinc being negative electrod and other(carbon/copper)being the positive electrode.
Primary cell has a perticular meaning, this being that the zinc dissolves slowly while the cell is functioning. Eventually, the zinc is eaten away to the extent that it prevents further operation of the cell. When this stage is reached, the cell has exhausted its useful life. Primary cell has a very limited life.
The Zinc-Carbon (Dry) Cell:
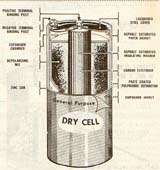 In this cell the positive electrode is made of carbon and negative electrode is made of zinc. The electrolyte is a chemical known as ammonium chloride(NH4CL), often called sal ammoniac. The negative electrode is in form of a cotainer and holds the entire cell. The positive element is in form of a carbon rod located at the center of the cell. A fully charged cell produces 1.6Volts.
In this cell the positive electrode is made of carbon and negative electrode is made of zinc. The electrolyte is a chemical known as ammonium chloride(NH4CL), often called sal ammoniac. The negative electrode is in form of a cotainer and holds the entire cell. The positive element is in form of a carbon rod located at the center of the cell. A fully charged cell produces 1.6Volts.